Early diagnosis and non-invasive monitoring of neurological disorders require sensitivity to elusive cellular-level alterations that emerge much earlier than volumetric changes observable with the millimetre-resolution medical imaging. Morphological changes in axons – the tube-like projections of neurons that transmit electrical signals and constitute the bulk of the brain’s white matter – are a common hallmark of a wide range of neurological disorders, as well as normal development and ageing. A recent study from the University of Eastern Finland (UEF) and the New York University (NYU) Grossman School of Medicine establishes a direct analytical link between the axonal microgeometry and non-invasive, millimetre-scale diffusion MRI (dMRI) signals – diffusion MRI measures the diffusion of water molecules within biological tissues and is sensitive to tissue microstructure.
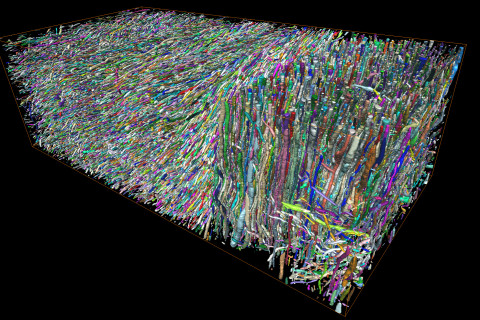
The discovery by Academy Research Fellow Dr Ali Abdollahzadeh at UEF and Professor Dmitry S. Novikov at NYU emerged from the convergence of two advances: unprecedented three-dimensional reconstruction of white matter microstructure using large-scale volume electron microscopy (vEM) and modern theoretical developments in diffusion physics. Advanced computational methods developed by Abdollahzadeh made it possible to reconstruct hundreds of thousands of axons with nanometre precision from vEM images acquired in Research Director Alejandra Sierra’s lab at UEF, establishing what is now the world’s largest quantitative three-dimensional reference of white matter microgeometry.
“These reconstructions revealed how axons deviate from simple straight tubes. We directly measured fluctuations in axonal cross-section and undulations along their length, among other morphological parameters,” says Abdollahzadeh.
During Abdollahzadeh’s postdoctoral research at NYU, led by Professors Dmitry S. Novikov and Els Fieremans, and building on Novikov’s pioneering advances in diffusion physics, Abdollahzadeh and Novikov developed a scattering framework for diffusion in cross-sectionally varying axons, yielding an exact solution to the Fick–Jacobs equation governing axial diffusion along axons.
“To solve a complex physical problem means identifying which parameters truly matter. Despite the infinite geometric complexity of axons, we found that only two structural parameters govern axial diffusion at experimentally accessible diffusion times: the average reciprocal cross-section and the variance of long-range cross-sectional fluctuations,” says Novikov.
Validation in an experimental rat model of traumatic brain injury at UEF’s Kuopio Biomedical Imaging Unit showed that ex vivo dMRI is sensitive to these microstructural parameters, even months after injury, transforming the interpretation of dMRI into a quantitative probe of axonal geometry.
These findings, now published in Nature Communications and selected as an Editors’ Highlight — “From Brain to Behaviour”, open new avenues for non-invasive biomarkers of white matter injury. Axonal shape changes are a hallmark of many neurological disorders, and this advance now enables their detection, monitoring and assessment of treatment response using dMRI.
Looking ahead, the team is extending this framework to animal models of diverse neurological pathologies at the Kuopio Biomedical Imaging Unit, headed by Professor Olli Gröhn, using the newly installed state-of-the-art 9.4-T MRI system. In parallel, translation to the human brain is advancing on human MRI scanners at Kuopio University Hospital and at NYU with the newly installed next-generation Connectom.X scanner with ultra-strong gradients.
“The combination of MRI in animal models and clinical MRI creates a translational path from nanometre-scale tissue microstructure to human neuroimaging, allowing us to test such microstructure-specific biomarkers in patients for the first time,” says Fieremans.
Research article:
Abdollahzadeh, A., Coronado-Leija, R., Lee, HH., Sierra, A., Fieremans, E., Novikov, D.S. Scattering approach to diffusion quantifies axonal damage in brain injury. Nat Commun16, 9808 (2025). https://doi.org/10.1038/s41467-025-64793-1