
Leaders
Group Overview
The Asquith Medicinal Chemistry Group was established in late 2021 based at the School of Pharmacy, part of the University of Eastern Finland. The lab explores novel chemical strategies to improve the efficacy and safety of viral and cancer treatments. This includes development of highly selective inhibitors of kinases involved in cancer and host directed anti-virals. The focus is on the translation of fundamental and clinical research findings to real world application through a series of cutting-edge medicinal chemistry tools including prognostic solvation shell mapping, free energy perturbation (FEP) calculations and machine learning algorithms. These techniques coupling with a strong synthetic background enable us to use advanced medicinal chemistry as a central framework to translate research from early-stage discovery, through optimization, to real-world clinical benefit.
We have been integrally involved in projects towards the development of chemical probes for the under-studied kinases including GAK, AAK1, BIKE, STK10, SLK, CaMKK2, PKN3, ULK3, ADCK3/COQ8A, MAP4K4, TLK2 and PKMYT1. The aim of these efforts in part is to generate research tools to enable the elucidation of the biological functions of these kinases. This has resulted in four high-quality chemical probes for GAK ( UNC-CA93.0 / SGC-GAK-1 ) [1-2], EGFR ( UNC-CA359 ) [3-4], PKN3 ( UNC-CA94 ) [ 5], COQ8A ( TPP-UNC-CA157 ) [6]. These efforts have also laid the groundwork for the development of other chemical tools and probes for kinases including MAP4K4 ( UNC-CA409 ) [7], STK10 & SLK [8] and more recently CaMKK2 [9] leading to SGC-CaMKK2-1, and TLK2 ( UNC-CA2-103 ) [10].
Selected References
- Asquith, CRM et al. Identification and Optimization of 4-Anilinoquinolines as Inhibitors of Cyclin G Associated Kinase. ChemMedChem. 2018 , 13 , (1), 48-66. doi: 10.1002/cmdc.201700663.
- Asquith, CRM et al. SGC-GAK-1: A Chemical Probe for Cyclin G Associated Kinase (GAK). J. Med. Chem. 2019 , 62 , (5), 2830-2836. doi: 10.1021/acs.jmedchem.8b01213.
- Asquith, CRM et al. Design of a Cyclin G Associated Kinase (GAK)/Epidermal Growth Factor Receptor (EGFR) Inhibitor Set to Interrogate the Relationship of EGFR and GAK in Chordoma. J Med Chem. 2019 , 62 , (9), 4772-4778. doi: 10.1021/acs.jmedchem.9b00350.
- Bieberich, AA et al. Optimization of the 4-anilinoquin(az)oline scaffold as epidermal growth factor receptor (EGFR) inhibitors for chordoma utilizing a Toxicology profiling assay platform. Sci Rep. 2022 , 12 , (1), 12820. doi: 10.1038/s41598-022-15552-5.
- Asquith, CRM et al. Identification of 4-Anilinoquin(az)oline as a Cell-Active Protein Kinase Novel 3 (PKN3) Inhibitor Chemotype. ChemMedChem. 2022 , 17 , (12), e202200161. doi: 10.1002/cmdc.202200161.
- Murray, NH et al. Small-molecule inhibition of the archetypal UbiB protein COQ8. Nat Chem Biol. 2023 , 19 , (2), 230-238. doi: 10.1038/s41589-022-01168-3.
- Strang, BL et al. Identification of lead anti-human cytomegalovirus compounds targeting MAP4K4 via machine learning analysis of kinase inhibitor screening data. PLoS One. 2018 , 13 , (7), e0201321. doi: 10.1371/journal.pone.0201321.
- Asquith, CRM et al. Design and Analysis of the 4-Anilinoquin(az)oline Kinase Inhibition Profiles of GAK/SLK/STK10 Using Quantitative Structure-Activity Relationships. ChemMedChem. 2020 , 15 , (1), 26-49. doi: 10.1002/cmdc.201900521.
- Eduful, BJ et al. Hinge Binder Scaffold Hopping Identifies Potent Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2) Inhibitor Chemotypes. J Med Chem. 2021 , 64 , (15), 10849-10877. doi: 10.1021/acs.jmedchem.0c02274.
- Asquith, CRM et al. Discovery and Optimization of Narrow Spectrum Inhibitors of Tousled Like Kinase 2 (TLK2) Using Quantitative Structure Activity Relationships. Eur J Med Chem. 2024 , 271 , 116357. doi: 10.1016/j.ejmech.2024.116357.
News
-
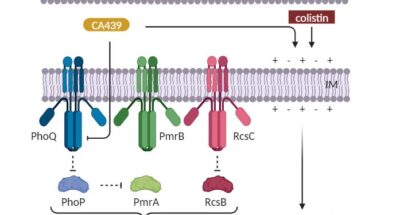 New dual therapeutic strategy shows promise against multidrug-resistant salmonella
New dual therapeutic strategy shows promise against multidrug-resistant salmonellaNew dual therapeutic strategy shows promise against multidrug-resistant salmonella
This story highlights our collaborative study leading to a new therapeutic strategy to target the multidrug-resistant bacterium Salmonella enterica in… -
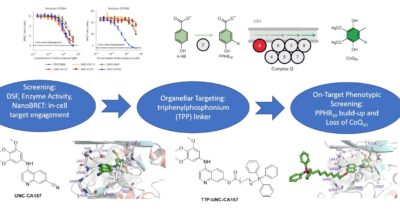 The first potent COQ8 inhibitor targets ubiquinone biosynthesis
The first potent COQ8 inhibitor targets ubiquinone biosynthesisThe first potent COQ8 inhibitor targets ubiquinone biosynthesis
This story highlights our collaborative study on the discovery and application of a new chemical probe to selectively inhibit human COQ8A in cells.
Cooperation
-
 Pantsar dynamics 01.10.2022 -
Pantsar dynamics 01.10.2022 - -
 Molecular Modeling and Drug Design Research Group 01.01.2010 -
Molecular Modeling and Drug Design Research Group 01.01.2010 - -
 Cancer Cell Plasticity - Ketola Lab 01.09.2019 -
Cancer Cell Plasticity - Ketola Lab 01.09.2019 -
Keywords
Leaders
Senior Researchers
Post-doctoral Researchers
Doctoral Researchers
-
Renne Leini
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences -
Arun Tonduru
Doctoral ResearcherSchool of Pharmacy, Faculty of Health Sciences
Technicians
Other group members
-
Juho Karuaho
-
Maja Pinhammer
Publications
30 items-
Enhancing colistin efficacy against Salmonella infections with a quinazoline-based dual therapeutic strategy
Lobertti, Carlos A; Gizzi, Fernán O; Magni, Christian; Rial, Analía; Chabalgoity, José A; Yim, Lucía; Blancato, Víctor S; Asquith, Christopher R M; García Véscovi, Eleonora. 2024. Scientific reports. 14: . 5148 -
The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold
Kalogirou, Andreas S; Oh, Hans J; Asquith, Christopher RM. 2023. Molecules. 28: -
Utilization of Supervised Machine Learning to Understand Kinase Inhibitor Toxophore Profiles
Bieberich, Andrew A; Asquith, Christopher RM. 2023. International journal of molecular sciences. 24: -
Identification of 4‐Anilinoquin(az)oline as a Cell‐Active Protein Kinase Novel 3 (PKN3) Inhibitor Chemotype**
Asquith, Christopher R. M.; Temme, Louisa; East, Michael P.; Laitinen, Tuomo; Pickett, Julie; Kwarcinski, Frank E.; Sinha, Parvathi; Wells, Carrow I.; Johnson, Gary L.; Zutshi, Reena; Drewry, David H.. 2022. Chemmedchem. 17: . e202200161 -
Numb-associated kinases are required for SARS-CoV-2 infection and are cellular targets for antiviral strategies
Karim, Marwah; Saul, Sirle; Ghita, Luca; Sahoo, Malaya Kumar; Ye, Chengjin; Bhalla, Nishank; Lo, Chieh-Wen; Jin, Jing; Park, Jun-Gyu; Martinez-Gualda, Belén; East, Michael Patrick; Johnson, Gary L; Pinsky, Benjamin A; Martinez-Sobrido, Luis; Asquith, Christopher RM; Narayanan, Aarthi; De Jonghe, Steven; Einav, Shirit. 2022. Antiviral research. 204: -
Optimization of the 4-anilinoquin(az)oline scaffold as epidermal growth factor receptor (EGFR) inhibitors for chordoma utilizing a toxicology profiling assay platform
Bieberich, Andrew A; Laitinen, Tuomo; Maffuid, Kaitlyn; Fatig, Raymond O; Torrice, Chad D; Morris, David C; Crona, Daniel J; Asquith, Christopher RM. 2022. Scientific reports. 12: . 12820 -
PKN3: a target in cancer metastasis
Temme, Louisa; Laitinen, Tuomo; Asquith, Christopher RM. 2022. Nature reviews drug discovery. 21: -
Small-molecule inhibition of the archetypal UbiB protein COQ8
Murray, Nathan H.; Asquith, Christopher R. M.; Fang, Zixiang; East, Michael P.; Ptak, Naomi; Smith, Robert W.; Vasta, James D.; Zimprich, Chad A.; Corona, Cesear R.; Robers, Matthew B.; Johnson, Gary L.; Bingman, Craig A.; Pagliarini, David J.. 2022. Nature chemical biology. 2023; 19: 230-238 -
Synthesis and evaluation of 1,2,3-dithiazole inhibitors of the nucleocapsid protein of feline immunodeficiency virus (FIV) as a model for HIV infection
Laitinen, Tuomo; Meili, Theres; Koyioni, Maria; Koutentis, Panayiotis A; Poso, Antti; Hofmann-Lehmann, Regina; Asquith, Christopher RM. 2022. Bioorganic and medicinal chemistry. 68: -
BCKDK: an emerging kinase target for metabolic diseases and cancer
East, Michael P; Laitinen, Tuomo; Asquith, Christopher RM. 2021. Nature reviews drug discovery. 20: 498


